PREECLAMPSIA

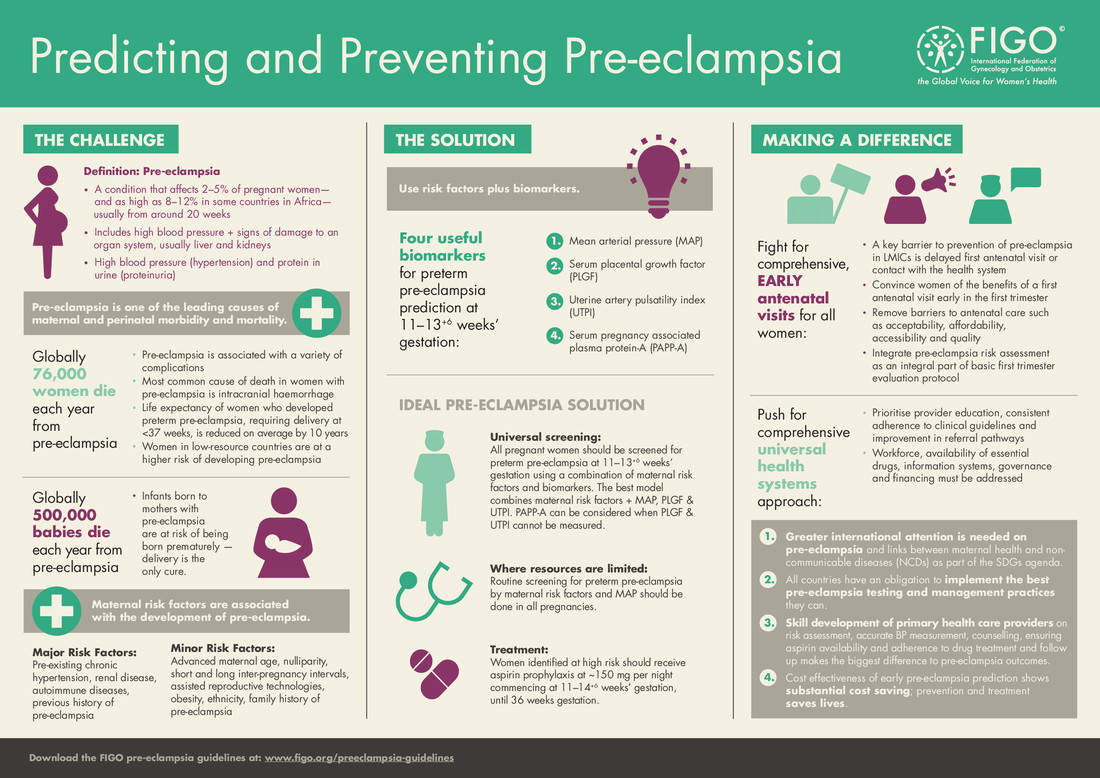
Although the mechanism of disease development is still not fully explained, it is assumed that in addition to a certain predisposition of a pregnant woman, an abnormal embryo implantation process plays the major role, resulting in damage to blood vessel structures manifesting itself in the pregnant woman with hypertension, damage to the function of many organs, including kidneys (proteinuria), liver (increased AST, ALT parameters), neurological and haematological complications. Fetal complications include growth retardation, oligohydramnios, low Apgar values and the need to admit the infant into the intensive care unit. Apart from adverse effects on the course of pregnancy, PE results in the risk of other complications, including the most serious ones. The disease is mentioned among the main causes of death of pregnant women and foetuses. In serious, untreatable situations, premature birth remains the only rescue for the mother and the foetus.
Maternal causes related to the development of PE include: first pregnancy, late age of pregnancy, history of PE, assisted reproductive technologies, obesity, pre-existing hypertension, kidney disease, autoimmune diseases (APS, SLE), hyperglycaemia.
Anticipating the risk of disease, its prevention and treatment is one of the most serious challenges of prenatal care today.
The current approach to early detection of PE is based on the identification of risk factors based on demographic data and the interview of the pregnant woman. An additional element of screening tests is the assessment of physical and biochemical biomarkers that enable the estimation of the individual risk for the patient. Intensive research carried out in recent years has identified several potentially useful markers: mean arterial pressure, uterine arterial pulsation index and biochemical markers detected in pregnant woman’s plasma – pregnancy-related protein (PAPP-A*) and placental growth factor (PLGF**).
* PAPP-A protein is a part of the biochemical screening test – the double test – performed as a standard procedure to increase the sensitivity of detecting the most common chromosomal aberrations.
** PLGF assessment is an additional biochemical test that can be performed simultaneously with the double test from the same blood sample.
The calculation of individual risk based on medical data, interview and marker assessment is currently the recommended method for detecting patients at risk of preeclampsia development during pregnancy.
Distinguishing the group with an increased risk of PE development at the beginning of pregnancy, long before the first clinical symptoms appear, enables the application of prophylaxis.
Numerous scientific studies have shown that acetylsalicylic acid (Acard, Polopyrin) taken before the 16th week of pregnancy significantly reduces the risk of preeclampsia.
According to the current state of medical knowledge and based on FIGO and PTGiP recommendations, there is no doubt that the most perfect method of prophylaxis is to include acetylsalicylic acid in the group of women at high risk calculated for a specific patient on the basis of biophysical and biochemical parameters.
Where a personalized PE risk assessment is available, the decision to use acetylsalicylic acid should be based on the estimated risk. In the case of PE risk higher than 1:150, it is recommended to include 100-150 mg of acetylsalicylic acid per day in one dose in the evening. It is necessary to start the therapy before week 16 of pregnancy. The drug shall be taken until week 36 of pregnancy.
REGISTRATION
Opening hours
Monday-Friday 8:00-20:00